Safety of complementary and alternative medicine products in children in Italy
Complementary and alternative medicine (CAM) includes dietary supplements, homeopathic products, herbal preparations, homemade and galenic preparations. These products are used to complement or as an alternative to conventional medications to prevent or treat diseases in the pediatric population.
A frequent use
Over the past decade, CAM use has increased in the population, accounting for a significant portion of treatment regimens in children as well.
Estimates of CAM use in adults range annually from 20% to 28% in the United Kingdom and 34% to 38% in the United States. A systematic review estimated a prevalence of CAM use in the global population of between 23% and 62%. Estimates of CAM use in children are also quite high, ranging from 11% to 51%. The variability in estimates depends on the definition of CAM and the study design. In children and adolescents, CAM is generally used to treat mild pain, respiratory tract conditions, anxiety or stress, attention deficit hyperactivity disorder, and sleep disorders. Of note, pediatric patients using CAM may also be suffering from chronic conditions, infections, and allergies.
In this context, the efficacy and safety of CAM remain relatively underestimated, and the potential for interactions (CAM-drug and/or CAM-disease), acute or chronic toxicity, or withdrawal that may be experienced by children to whom CAM is administered concurrently with or in place of their conventional medications is easily imagined. In the scientific literature, in fact, the number of reports of suspected adverse reactions associated to the use of CAM has significantly increased in recent years.
The situation in Italy
In Italy, as in other high-income countries, the range of CAM used to treat children is very heterogeneous and may include self-medication products, treatments requiring consultation with qualified healthcare professionals and/or the use of herbal or homeopathic products.
Only a few surveys have estimated the prevalence of CAM users in Italy (13% to 15%), reporting that homeopathy is by far the most widely used CAM, followed by phytotherapy and acupuncture. These treatments are more common in Northern Italy. In Tuscany, a region where CAM is widely used and where it is partially reimbursed by the Regional Health Service, the prevalence of CAM users is 15-20%, and 45% of the interviewed population considers useful at least one type of CAM.
In Italy, as in many other countries, CAM, in particular dietary supplements, are notified and not registered as conventional drugs and therefore their safety profile is not known before their release on the market.
Since 2002 in Italy spontaneous reports of suspected adverse reactions potentially related to CAM are collected within the Italian System of Phytovigilance, coordinated by the Istituto Superiore di Sanità (ISS).
The Italian study
The aim of an Italian study published in 2019 in Phytomedicine was to analyze (in terms of frequency and severity) the suspected adverse reactions associated with the use of CAM in the pediatric population (0-18 years), identifying the presence of potential factors associated with their severity.1 This work was carried out in collaboration between the Pharmacovigilance, Phytovigilance and Pharmacoepidemiology Research Unit of the University of Florence, the Centro di Riferimento Regionale in Fitoterapia (CERFIT) of the AOU Careggi of Florence and ISS. The study is the result of a complex and in-depth retrospective analysis (16 years) of reports of suspected adverse reactions to CAM products used in the pediatric population (0-18 years), received by ISS. Each single report of adverse reaction was examined and data were collected on the demographic and clinical characteristics of the children, the suspected CAM(s), conventional drugs and concomitant diseases, with the aim of identifying potential predictors of the severity of adverse reactions. The complexity of the content of the products analyzed did not allow us to evaluate the products on the basis of their specific composition, that is, considering the content of each individual product. For this reason, analyses were performed by “clusters”, i.e. on the basis of the type of product (e.g., supplement, homeopathic, etc.) and the total number of active components contained.
In total, 206 reports of adverse reactions related to children were analyzed. Sixty-nine reports were defined as “serious2 (33.5%) and of these 59 resulted in access to the emergency department and/or hospitalization. No reports of adverse reactions were associated with death.
Most reports were of male, Caucasian children with a mean age of 36 months. A total of 105 (50.97%) adverse reaction reports had complete resolution and 27 (13.11%) had improvement. For 59 (28.64%) reports, outcome data were not available.
Overall, patients were using only one CAM when the adverse reaction occurred (n=193, 93.69%) and most were not using other concomitant medications (n=154, 74.76%). Eighty adverse reaction reports (38.83%) were for suspected products containing 2 to 5 active components. This frequency was highest for reports of adverse reactions defined as “serious” (n=31, 41.89%).
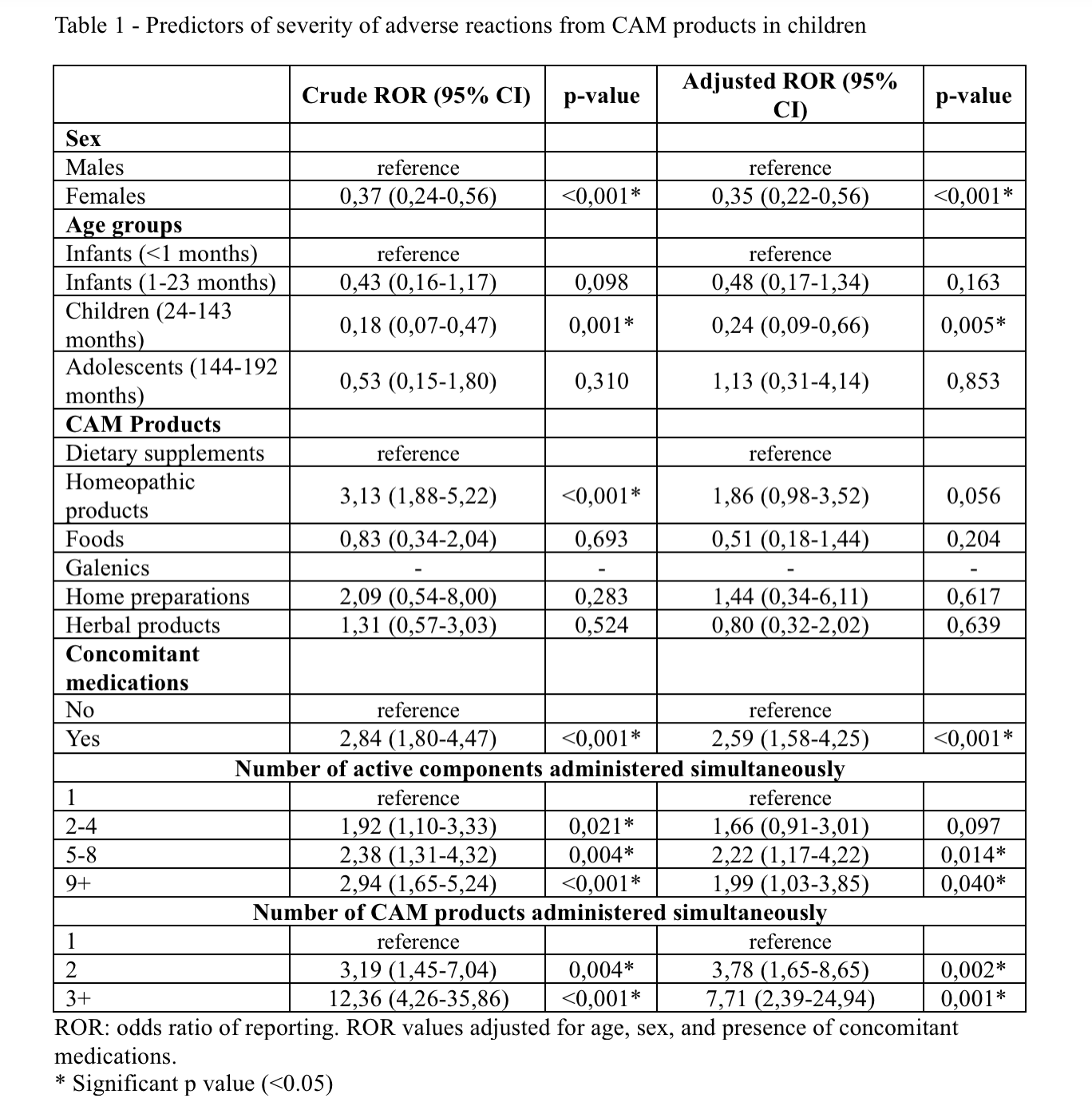
Most of the reported adverse reactions were related to dietary supplement use (57.18%), and the SOC (System Organ Class) primarily involved was skin and subcutaneous tissue (40.29%). As reported in Table 1, the occurrence of serious adverse reactions was 3 times higher in children exposed to homeopathic products (crude ROR 3.13, 95% confidence limits 1.88 to 5.22), 2.5 times higher in children exposed to CAM in the presence of concomitant medications (adjusted ROR 2.59, 95% confidence limits 1.58 to 4.25), slightly more than 2-fold higher in children exposed to CAM containing 5 to 8 components (adjusted ROR 2.22, 95% confidence limits 1.17 to 4.22), and nearly 8-fold higher in children exposed to more than three CAMs simultaneously (adjusted ROR 7.71, 95% confidence limits 2.39 to 24.94). A very interesting result was that relating to homeopathic products, for which a higher risk of serious adverse reactions was shown in our sample. This could be explained by their ineffectiveness in treating acute or chronic diseases and their consequent worsening, rather than by their direct toxicity.
On the basis of the evidence available in the literature, this study represents the largest phytovigilance analysis carried out in Italy in recent years and provides new information on factors that could increase the risk of serious adverse reactions associated with the use of CAM in children.
Conclusions
In particular, healthcare professionals (physicians, pharmacists, etc.) should always keep in mind that CAM products containing more than five components and administered concurrently with conventional medications may increase, in a statistically significant manner, the risk of developing serious adverse reactions in children.
It is certain that further studies in populations, particularly pediatric populations, are needed to evaluate the efficacy and safety of CAM, its potential effect following long-term use, and also the possible interactions between CAM and concomitant drugs/diseases.
In any case, our work confirms that the Italian System of Phytovigilance represents to date the best strategy to estimate and characterize the clinical impact of adverse reactions to CAM products in real clinical practice, with the ultimate goal to improve the appropriateness of use of these products in the general population, especially in children.
We remind all italian readers that through the portal VigiErbe (www.vigierbe.it) it is possible to report online suspected adverse reactions occurring after the intake of dietary supplements, herbal products, magistral preparations (e.g. based on cannabis for medical use), homeopathic products (not registered as medicines) and other products of natural origin. The VigiErbe website has been active since December 12, 2018, and replaces the old paper reporting via fax. All reports are collected in a single database at ISS, which is also entrusted with coordination functions.
Niccolò Lombardi1,2, Giada Crescioli1,2, Alfredo Vannacci1,2
1 Research Unit in Pharmacovigilance, Phytovigilance and Pharmacoepidemiology, Pharmacology and Toxicology Section, Department of Neuropharmacy, University of Florence, Florence
2. Regional Center of Pharmacovigilance of Tuscany, USL Toscana Centro, Florence
- Lombardi N, Crescioli G, Bettiol A, Menniti-Ippolito F, Maggini V, Gallo E, Mugelli A, Vannacci A, Firenzuoli F. Safety of complementary and alternative medicine in children: a 16-years retrospective analysis of the Italian Phytovigilance system database. Phytomedicine 2019;61:152856. CDI